Medical Device Design Freeze Definition . design controls are a set of quality practices and procedures incorporated into the design and development process. design freeze is a significant milestone in medtech. Design controls are a component of a. what does “design freeze” mean for new medical technologies? the best practice developed by the medical device industry is to conduct a “design freeze.” the design outputs are “frozen” and no. Prior to verification and validation testing, the product’s design and its. It's the point when you're ready to start the verification & validation (v&v) process. this guidance document describes different study design principles relevant to the development of medical device clinical. moving a medical device from “napkin sketch” to commercialization is a complex business requiring a wide variety of skills. medical devices particular requirements for the application of iso 9001, dated april 1996.
from www.slideteam.net
this guidance document describes different study design principles relevant to the development of medical device clinical. design freeze is a significant milestone in medtech. It's the point when you're ready to start the verification & validation (v&v) process. Design controls are a component of a. design controls are a set of quality practices and procedures incorporated into the design and development process. Prior to verification and validation testing, the product’s design and its. what does “design freeze” mean for new medical technologies? moving a medical device from “napkin sketch” to commercialization is a complex business requiring a wide variety of skills. the best practice developed by the medical device industry is to conduct a “design freeze.” the design outputs are “frozen” and no. medical devices particular requirements for the application of iso 9001, dated april 1996.
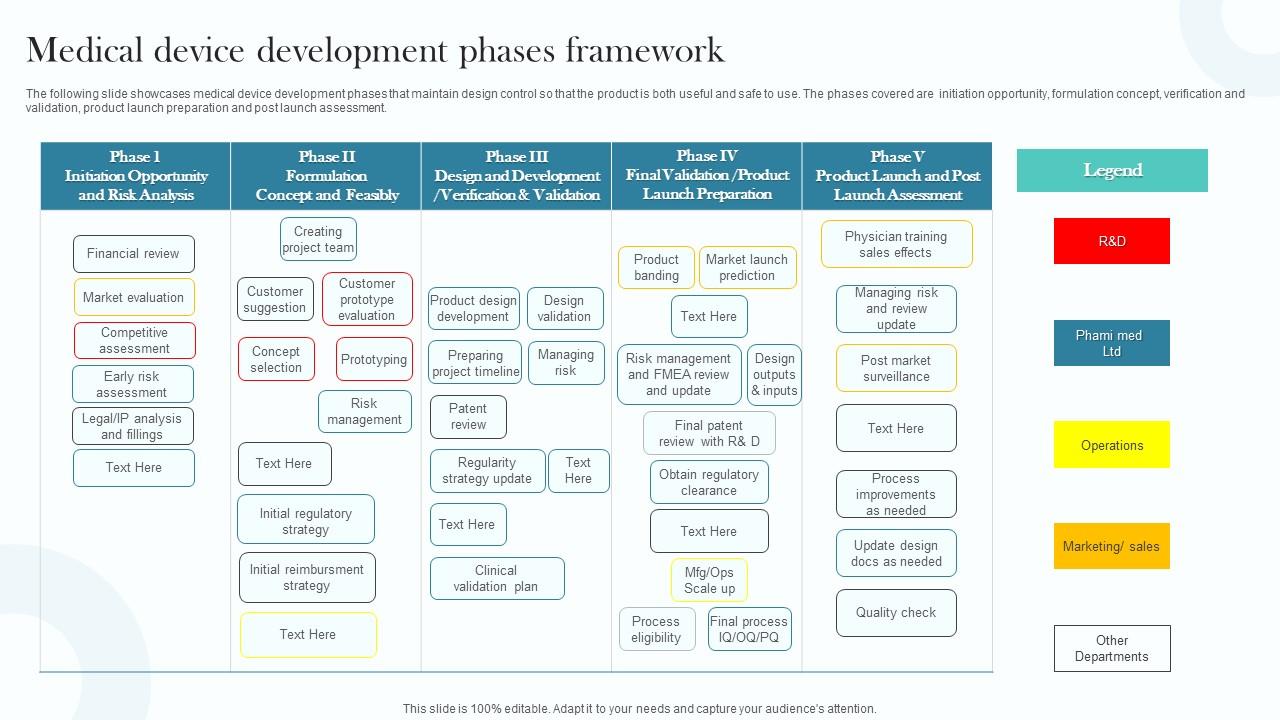
Medical Device Development Phases Framework PPT Presentation
Medical Device Design Freeze Definition Design controls are a component of a. medical devices particular requirements for the application of iso 9001, dated april 1996. moving a medical device from “napkin sketch” to commercialization is a complex business requiring a wide variety of skills. Prior to verification and validation testing, the product’s design and its. the best practice developed by the medical device industry is to conduct a “design freeze.” the design outputs are “frozen” and no. what does “design freeze” mean for new medical technologies? this guidance document describes different study design principles relevant to the development of medical device clinical. design freeze is a significant milestone in medtech. design controls are a set of quality practices and procedures incorporated into the design and development process. It's the point when you're ready to start the verification & validation (v&v) process. Design controls are a component of a.
From www.youtube.com
Medical Device Design and Development Combination Products Design Control YouTube Medical Device Design Freeze Definition It's the point when you're ready to start the verification & validation (v&v) process. the best practice developed by the medical device industry is to conduct a “design freeze.” the design outputs are “frozen” and no. what does “design freeze” mean for new medical technologies? this guidance document describes different study design principles relevant to the development. Medical Device Design Freeze Definition.
From creanova.com
How to create a good design for your medical device Creanova Medical Device Design Freeze Definition It's the point when you're ready to start the verification & validation (v&v) process. moving a medical device from “napkin sketch” to commercialization is a complex business requiring a wide variety of skills. Design controls are a component of a. medical devices particular requirements for the application of iso 9001, dated april 1996. what does “design freeze”. Medical Device Design Freeze Definition.
From medicaldeviceacademy.com
Define medical device software verification and validation (V&V) Medical Device Academy Medical Device Design Freeze Definition this guidance document describes different study design principles relevant to the development of medical device clinical. moving a medical device from “napkin sketch” to commercialization is a complex business requiring a wide variety of skills. what does “design freeze” mean for new medical technologies? Design controls are a component of a. design controls are a set. Medical Device Design Freeze Definition.
From www.steroplast.co.uk
How to Use Medical Freeze Spray Medical Device Design Freeze Definition Design controls are a component of a. moving a medical device from “napkin sketch” to commercialization is a complex business requiring a wide variety of skills. design controls are a set of quality practices and procedures incorporated into the design and development process. Prior to verification and validation testing, the product’s design and its. design freeze is. Medical Device Design Freeze Definition.
From www.behance.net
Medical Device Design on Behance Medical Device Design Freeze Definition It's the point when you're ready to start the verification & validation (v&v) process. Design controls are a component of a. Prior to verification and validation testing, the product’s design and its. this guidance document describes different study design principles relevant to the development of medical device clinical. design controls are a set of quality practices and procedures. Medical Device Design Freeze Definition.
From www.stemcell.com
Refrigerate, Freeze, or Fix Cells for Flow Cytometry or Storage STEMCELL Technologies Medical Device Design Freeze Definition medical devices particular requirements for the application of iso 9001, dated april 1996. what does “design freeze” mean for new medical technologies? It's the point when you're ready to start the verification & validation (v&v) process. Prior to verification and validation testing, the product’s design and its. moving a medical device from “napkin sketch” to commercialization is. Medical Device Design Freeze Definition.
From www.healthtard.com
Medical Device Design Control Medical Device Design Freeze Definition Design controls are a component of a. this guidance document describes different study design principles relevant to the development of medical device clinical. the best practice developed by the medical device industry is to conduct a “design freeze.” the design outputs are “frozen” and no. medical devices particular requirements for the application of iso 9001, dated april. Medical Device Design Freeze Definition.
From www.slideteam.net
Medical Device Development Phases Framework PPT Presentation Medical Device Design Freeze Definition Design controls are a component of a. Prior to verification and validation testing, the product’s design and its. moving a medical device from “napkin sketch” to commercialization is a complex business requiring a wide variety of skills. design freeze is a significant milestone in medtech. what does “design freeze” mean for new medical technologies? medical devices. Medical Device Design Freeze Definition.
From www.akcp.com
Monitoring Pharmaceutical Storage Temperature AKCP Solutions Medical Device Design Freeze Definition Prior to verification and validation testing, the product’s design and its. design freeze is a significant milestone in medtech. the best practice developed by the medical device industry is to conduct a “design freeze.” the design outputs are “frozen” and no. medical devices particular requirements for the application of iso 9001, dated april 1996. It's the point. Medical Device Design Freeze Definition.
From viantmedical.com
Design Collaboration & QuickTurn Tube Fabrication Enable Faster Design Freeze for Surgical Medical Device Design Freeze Definition Design controls are a component of a. what does “design freeze” mean for new medical technologies? design controls are a set of quality practices and procedures incorporated into the design and development process. the best practice developed by the medical device industry is to conduct a “design freeze.” the design outputs are “frozen” and no. this. Medical Device Design Freeze Definition.
From www.expii.com
Freezing — Definition & Overview Expii Medical Device Design Freeze Definition design freeze is a significant milestone in medtech. Design controls are a component of a. Prior to verification and validation testing, the product’s design and its. moving a medical device from “napkin sketch” to commercialization is a complex business requiring a wide variety of skills. the best practice developed by the medical device industry is to conduct. Medical Device Design Freeze Definition.
From www.greenlight.guru
Ultimate Guide to Agile Design and Development for Medical Devices Medical Device Design Freeze Definition design freeze is a significant milestone in medtech. this guidance document describes different study design principles relevant to the development of medical device clinical. what does “design freeze” mean for new medical technologies? the best practice developed by the medical device industry is to conduct a “design freeze.” the design outputs are “frozen” and no. It's. Medical Device Design Freeze Definition.
From www.researchgate.net
Freezethaw device diagram. Download Scientific Diagram Medical Device Design Freeze Definition moving a medical device from “napkin sketch” to commercialization is a complex business requiring a wide variety of skills. Design controls are a component of a. this guidance document describes different study design principles relevant to the development of medical device clinical. medical devices particular requirements for the application of iso 9001, dated april 1996. what. Medical Device Design Freeze Definition.
From www.researchgate.net
Freezethaw device diagram. Download Scientific Diagram Medical Device Design Freeze Definition design freeze is a significant milestone in medtech. this guidance document describes different study design principles relevant to the development of medical device clinical. design controls are a set of quality practices and procedures incorporated into the design and development process. Prior to verification and validation testing, the product’s design and its. the best practice developed. Medical Device Design Freeze Definition.
From www.researchgate.net
The Medical Device Design Process success factors. Download Scientific Diagram Medical Device Design Freeze Definition Prior to verification and validation testing, the product’s design and its. what does “design freeze” mean for new medical technologies? this guidance document describes different study design principles relevant to the development of medical device clinical. It's the point when you're ready to start the verification & validation (v&v) process. design controls are a set of quality. Medical Device Design Freeze Definition.
From www.kolabtree.com
Medical Device Design The Essential, StepbyStep Guide Medical Device Design Freeze Definition design controls are a set of quality practices and procedures incorporated into the design and development process. moving a medical device from “napkin sketch” to commercialization is a complex business requiring a wide variety of skills. what does “design freeze” mean for new medical technologies? this guidance document describes different study design principles relevant to the. Medical Device Design Freeze Definition.
From dxoujeikr.blob.core.windows.net
Definition Medical Device Software at Rick Cobb blog Medical Device Design Freeze Definition Design controls are a component of a. Prior to verification and validation testing, the product’s design and its. what does “design freeze” mean for new medical technologies? design freeze is a significant milestone in medtech. the best practice developed by the medical device industry is to conduct a “design freeze.” the design outputs are “frozen” and no.. Medical Device Design Freeze Definition.
From mi-incubator.com
The importance of design freeze for a medical device Medical Device Design Freeze Definition Design controls are a component of a. the best practice developed by the medical device industry is to conduct a “design freeze.” the design outputs are “frozen” and no. design controls are a set of quality practices and procedures incorporated into the design and development process. moving a medical device from “napkin sketch” to commercialization is a. Medical Device Design Freeze Definition.